Increased level of malondialdehyde in preterm labor
Abstract
Background: The pathophysiological mechanism associated with spontaneous preterm delivery is oxidative stress through the increased formation of reactive oxygen species (ROS) due to lipid peroxidation. Malondialdehyde (MDA) is one of the biomarkers of oxidative stress produced through the lipid peroxidation process.
Objective: The aim of this study is to observe the difference in MDA levels among preterm labor compared to full-term labor.
Methods: Observational research was conducted with a comparative cross-sectional design. Maternal venous blood samples were taken from private hospitals and midwives in Padang city and Aro Suka Hospital Solok Regency. Samples were selected by consecutive sampling and divided into two groups with a total of 40 samples. MDA level was measured using the spectrophotometry method.
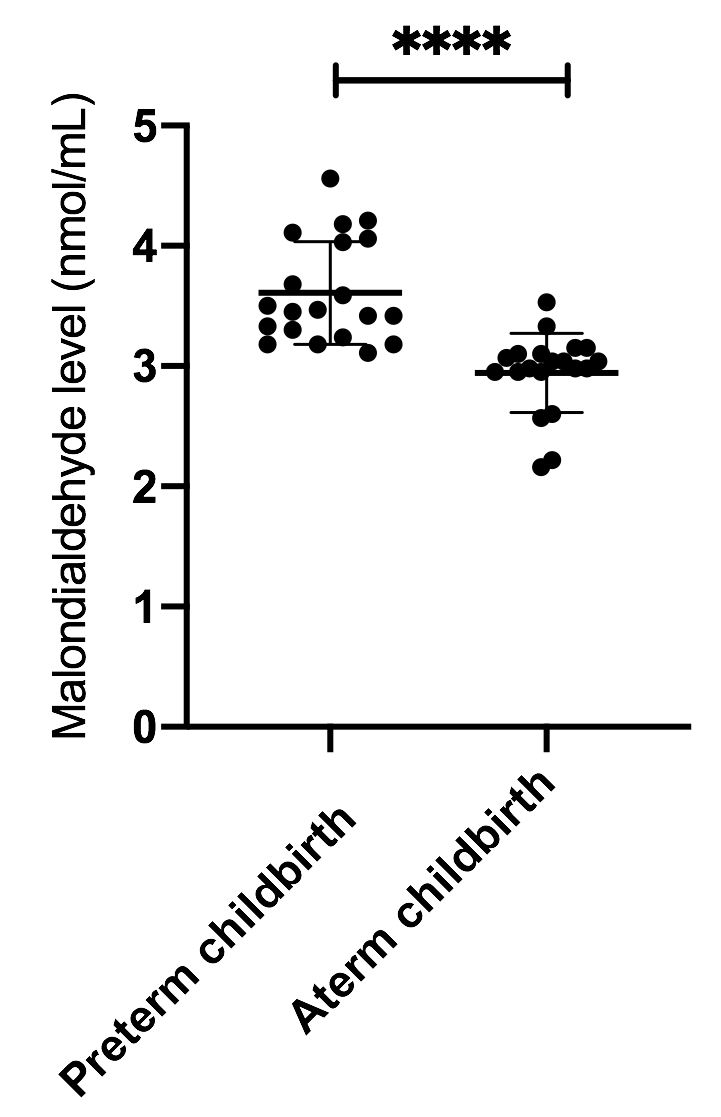
Results: MDA levels in preterm delivery were 3,6±0.42 nmol/mL and in full-term delivery were 2.9±0.33 nmol/mL.
Conclusion: There was a significant difference in MDA levels between preterm labor and full-term delivery. MDA levels in preterm childbirth were higher than MDA levels in full-term delivery.
References
WHO | New global estimates on preterm birth published [Internet]. [cited 21 Oct 2021]. Available: https://www.who.int/reproductivehealth/global-estimates-preterm-birth/en/
Laporan Hasil Riset Kesehatan Dasar (Riskesdas) | Badan Penelitian dan Pengembangan Kesehatan [Internet]. [cited 21 Oct 2021]. Available: https://www.litbang.kemkes.go.id/laporan-riset-kesehatan-dasar-riskesdas/
Alamrani A, Mahmoud S, Alotaibi M. Intrauterine infection as a possible trigger for labor: the role of toll-like receptors and proinflammatory cytokines. Asian Biomedicine. 2015;
Catov JM, Nohr EA, Olsen J, Ness RB. Chronic hypertension related to risk for preterm and term small for gestational age births. Obstet Gynecol. 2008;112: 290-296. https://doi.org/10.1097/AOG.0b013e31817f589b
Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med. 2012;17: 12-19. https://doi.org/10.1016/j.siny.2011.09.001
Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A, Extremely Low Gestational Age Newborns (ELGAN) Study Investigators. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198: 110.e1-7. https://doi.org/10.1016/j.ajog.2007.05.044
Shynlova O, Lee Y-H, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20: 154-167. https://doi.org/10.1177/1933719112446084
Lozovoy MAB, Simão ANC, Panis C, Rotter MAC, Reiche EMV, Morimoto HK, et al. Oxidative stress is associated with liver damage, inflammatory status, and corticosteroid therapy in patients with systemic lupus erythematosus. Lupus. 2011;20: 1250-1259. https://doi.org/10.1177/0961203311411350
Tanhapour M, Vaisi-Raygani A, Bahrehmand F, Khazaei M, Kiani A, Rahimi Z, et al. Association between the cytotoxic T-lymphocyte antigen-4 mutations and the susceptibility to systemic lupus erythematosus; Contribution markers of inflammation and oxidative stress. Cell Mol Biol (Noisy-le-grand). 2016;62: 56-61. doi:10.14715/cmb/2016.62.12.10
Hardt U, Larsson A, Gunnarsson I, Clancy RM, Petri M, Buyon JP, et al. Autoimmune reactivity to malondialdehyde adducts in systemic lupus erythematosus is associated with disease activity and nephritis. Arthritis Res Ther. 2018;20: 36. https://doi.org/10.1186/s13075-018-1530-2
Ramana KV, Srivastava S, Singhal SS. Lipid peroxidation products in human health and disease 2014. Oxid Med Cell Longev. 2014;2014: 162414. https://doi.org/10.1155/2014/162414
Shah D, Mahajan N, Sah S, Nath SK, Paudyal B. Oxidative stress and its biomarkers in systemic lupus erythematosus. J Biomed Sci. 2014;21: 23. https://doi.org/10.1186/1423-0127-21-23
Sangeetha Lakshmi B, Harini Devi N, Suchitra MM, Srinivasa Rao PVLN, Siva Kumar V. Changes in the inflammatory and oxidative stress markers during a single hemodialysis session in patients with chronic kidney disease. Ren Fail. 2018;40: 534-540. https://doi.org/10.1080/0886022X.2018.1487857
Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014: 360438. https://doi.org/10.1155/2014/360438
Mihailovič M, Cvetkovč M, Ljubič A, Kosanovič M, Nedeljkovič S, Jovanovič I, et al. Selenium and malondialdehyde content and glutathione peroxidase activity in maternal and umbilical cord blood and amniotic fluid. Biol Trace Elem Res. 2000;73: 47-54. https://doi.org/10.1385/BTER:73:1:47
El-Sayed AM, Galea S. Temporal changes in socioeconomic influences on health: maternal education and preterm birth. Am J Public Health. 2012;102: 1715-1721. https://doi.org/10.2105/AJPH.2011.300564
Slavić M, Appiah I, Nikolić-Kokić A, Radojicić R, Jones DR, Spasić MB, et al. The anti-oxidative defence system in the isolated rat uterus during spontaneous rhythmic activity. Acta Physiol Hung. 2006;93: 335-339. https://doi.org/10.1556/APhysiol.93.2006.4.9
Acton BM, Jurisicova A, Jurisica I, Casper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod. 2004;10: 23-32. https://doi.org/10.1093/molehr/gah004
Ludwig TE, Squirrell JM, Palmenberg AC, Bavister BD. Relationship between development, metabolism, and mitochondrial organization in 2-cell hamster embryos in the presence of low levels of phosphate. Biol Reprod. 2001;65: 1648-1654. https://doi.org/10.1095/biolreprod65.6.1648
Trimarchi JR, Liu L, Porterfield DM, Smith PJ, Keefe DL. Oxidative phosphorylation-dependent and -independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod. 2000;62: 1866-1874. https://doi.org/10.1095/biolreprod62.6.1866
Sultana Z, Maiti K, Aitken J, Morris J, Dedman L, Smith R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol. 2017;77. https://doi.org/10.1111/aji.12653
Martin A, Faes C, Debevec T, Rytz C, Millet G, Pialoux V. Preterm birth and oxidative stress: Effects of acute physical exercise and hypoxia physiological responses. Redox Biol. 2018;17: 315-322. https://doi.org/10.1016/j.redox.2018.04.022
Aponte A, Agarwal A. Premature rupture of membranes and oxidative stress. In: Agarwal A, Aziz N, Rizk B, editors. Studies on women's health. Totowa, NJ: Humana Press; 2013. pp. 143-147. https://doi.org/10.1007/978-1-62703-041-0_8
Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Behrman RE, Butler AS, editors. Washington (DC): National Academies Press (US); 2007. https://doi.org/10.17226/11622
Wu F, Tian F-J, Lin Y. Oxidative stress in placenta: health and diseases. Biomed Res Int. 2015;2015: 293271. https://doi.org/10.1155/2015/293271
Ali ME, Rahman MM, Sarkar SM, Hamid SBA. Heterogeneous metal catalysts for oxidation reactions. J Nanomater. 2014;2014: 1-23. https://doi.org/10.1155/2014/192038
Negi R, Pande D, Kumar A, Khanna RS, Khanna HD. In vivo oxidative DNA damage and lipid peroxidation as a biomarker of oxidative stress in preterm low-birthweight infants. J Trop Pediatr. 2012;58: 326-328. https://doi.org/10.1093/tropej/fmr078
Copyright (c) 2021 Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.







