Changes on oxidative stress-related biomarkers in plasma and cardiac tissue due to prolonged exposure to normobaric hyperoxia
Abstract
Background: Hyperoxia is a state of oversupply of oxygen in tissues and organs that can increase reactive oxygen species (ROS). When antioxidants cannot balance ROS levels, oxidative stress occurs. Catalase and reduced glutathione (GSH) are two of the antioxidants that can be very useful to counteract ROS. Increased production of ROS subsequently results in lipids damage and generates malondialdehyde (MDA). ROS interaction with cardiac cells causes remodeling thus leads to heart failure.
Objectives: The purpose of this study was to find out the changes on oxidative stress-related biomarkers in plasma and cardiac tissue.
Methods: Sprague Dawley rats were divided into 5 groups (n=6/group). Control group was exposed to normoxia (21% O2), while each treatment group was exposed to hyperoxia (75% O2) for 1, 3, 7, and 14 days. Blood and heart samples were used for blood gas analysis and hematology test, also for catalase specific activity measurement, GSH level, and MDA level measurement.
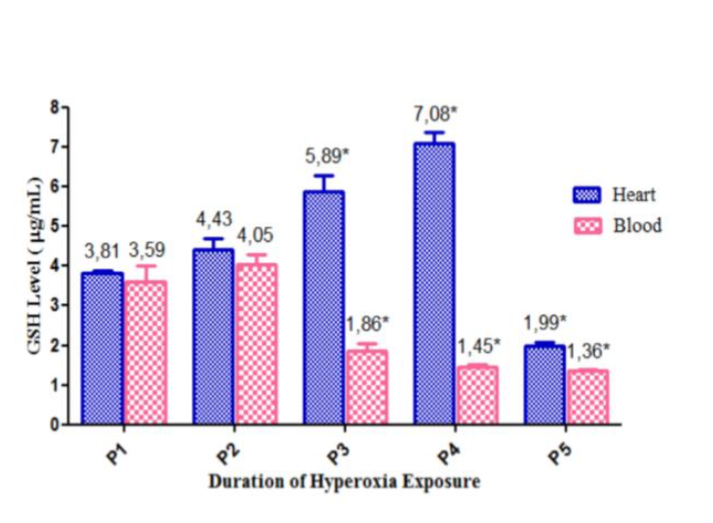
Results: Blood gas analysis of pO2, pCO2, and HCO3 were increased, while the O2 saturation and all hematological parameters were decreased. Plasma and cardiac tissue’s catalase specific activity increased in day 1 to day 7 but declined in day 14. Cardiac tissue’s GSH has the same result. Plasma GSH level increased in day 1 but decreased afterward. MDA level in plasma and cardiac tissue increased significantly since day 1.
Conclusion: Hyperoxia causes oxidative stress, marked by the increase of oxidative stress-related markers, and partially compensated respiratory acidosis.
References
Sherwood L. Human physiology: from cells to systems. 6th Ed. Jakarta: EGC; 2011.
Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. The Journal of the American Medical Association. 2010;303(21):2165-71. https://doi.org/10.1001/jama.2010.707
Lau YY, Tay YY, Shah VA, Chang P, Loh KT. Maintaining optimal oxygen saturation in premature infants. The Permanente Journal. 2011;15(1):108-13.
Farquhar H, Weatherall M, Wijesinghe M, Perrin K, Ranchord A, Simmonds M, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J. 2009;158(3): 371-7. https://doi.org/10.1016/j.ahj.2009.05.037
Dean JB, Mulkey DK, Henderson RA, Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brain stem neurons. J Appl Physiol. 2004;96(2):784-91. https://doi.org/10.1152/japplphysiol.00892.2003
Vincent JL, Taccone FS, He X. Harmful effects of hyperoxia in postcardiac arrest, sepsis, traumatic brain injury, or stroke: The importance of individualized oxygen therapy in critically ill patients. Canadian Respiratory Journal. 2017;2017:1-7. https://doi.org/10.1155/2017/2834956
Gonzales RR, Barrasa MJL, Nuez RA,Pedrosa CAM, Saavedra MMT,Bello GMA, et al. Multiple system organ response induced by hyperoxia in a clinically relevant animal model of sepsis. Shock. 2014;42(2):148-53. https://doi.org/10.1097/SHK.0000000000000189
Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity. 2014;2014:1-31. https://doi.org/10.1155/2014/360438
Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. The J of Clin Investigation. 2005;115(3):500-8. https://doi.org/10.1172/JCI200524408
Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. Journal of Biomedicine and Biotechnology. 2012;2012:1-14. https://doi.org/10.1155/2012/936486
Matés JM. Effect of antioxidant enzymes in the molecular control of rective oxygen species toxicology. Toxicology. 2000;153(1-3):83-104. https://doi.org/10.1016/S0300-483X(00)00306-1
Halliwell B, Gutteridge JM. Free radicals in biology and medicine. 5th ed. New York: Oxford University Press; 2015. https://doi.org/10.1093/acprof:oso/9780198717478.001.0001
Taverne YJ, Bogers AJ, Duncker DJ, Merkus D. Reactive oxygen species and the cardiovascular system. Oxidative Medicine and Cellular Longevity. 2013;2013:1-15. https://doi.org/10.1155/2013/862423
Moradkhan R, Sinoway LI. Revisiting the role of oxygen therapy in cardiac patients. J Am Coll Cardiol. 2010;56(13):1013-6. https://doi.org/10.1016/j.jacc.2010.04.052
Roy S, Khanna S, Wallace WA, Lappalainen J, Rink C, Cardounel AJ, et al. Characterization of perceived hyperoxia in isolated primary cardiac fibroblasts and in the reoxygenated heart. J Biol Chem. 2003;278(47):47129-35. https://doi.org/10.1074/jbc.M308703200
V Marina M, V Evgeniya K. Special features of deviations and correction pro and antioxidant blood plasma balance of rats under stress of different genesis. Advance Research in Scientific Areas. 2012;7(15):2158-60.
Rostami E, Rocksen D, Ekberg NR, Giony M, Ungerstedt U. Brain metabolism and oxygenation in healthy pigs receiving hypoventilation and hyperoxia. Respir Physiol Neurobiol. 2013;189(3):537-42. https://doi.org/10.1016/j.resp.2013.08.010
Byrd RP. Respiratory acidosis. [updated 2015 Jul 31, cited 2015 Dec 3]. Available from: http://emedicine.medscape.com/article/301574-overview#a7
Federer WT. Experimental design: theory and application. New York: Oxford & IBH Publishing Company; 1955.
Siantur M. Penentuan besar sampel. Semarang: Universitas Diponegoro; 2007.
Matés J, Pérez-Gómez C, De Castro I. Antioxidant enzymes and human diseases. Clinical Biochemistry. 1999; 32(8):595-603. https://doi.org/10.1016/S0009-9120(99)00075-2
Wills ED. Evaluation of lipid peroxidation in lipids and biological membranes. In: Snell K, Mullock B, editors. Biochemical toxicology: A practical approach. Oxford: IRL;1987:127-52.
Miguel F, Augusto AC, Gurgueira SA. Effect of acute vs chronic H2O2-induced oxidative stress on antioxidant enzyme activities. Free Radical Research. 2009;43(4):340-7. https://doi.org/10.1080/10715760902751894
Marina V, Evgeniya V. Special features of deviations and correction pro and antioxidant blood plasma balance of rats under stress of different genesis. Advance Research in Scientific Areas. 2012;7(15):2158-60.
Malmezat T, Breuille D, Capitan P, Mirand PP, Obled C. Glutathione turnover is increased during the acute phase of sepsis in rats. The Journal of Nutrition. 2000; 130(5):1239-46. https://doi.org/10.1093/jn/130.5.1239
Loiseaux-Meunier MN, Bedu M, Gentou C, Pepin D, Coudert J, Caillaud D. Oxygen toxicity: simultaneous measure of pentane and malondialdehyde in humans exposed to hyperoxia. Biomed Pharmacother. 2001;55(3):163-9. https://doi.org/10.1016/S0753-3322(01)00042-7
Sifringer M, Haefen CV, Krain M, Paeschke N, Bendix I, Buhrer C, et al. Neuroprotective effect of dexmedetomidine on hyperoxia-induced toxicity in the neonatal rat brain. Oxidative Medicine and Cellular Longevity.2015;2015:1-10. https://doi.org/10.1155/2015/530371
Bandali KS, Belanger MP, Wittnich C. Hyperoxia causes oxygen free radical-mediated membrane injury and alters myocardial function and hemodynamics in the newborn. Am J Physiol Heart Circ Physiol. 2004;287(2):553-9. https://doi.org/10.1152/ajpheart.00657.2003
Copyright (c) 2019 Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.







