Effect of glucose on reduced glutathione level in Malay uncomplicated type 2 diabetes patients
Abstract
Background: Increasing blood sugar level may increase free radical compounds in type 2 diabetes. Free radical compounds can cause oxidative stress, thereby decreasing endogenous antioxidants such as reduced glutathione (GSH).
Objective: This study aimed to determine whether random blood glucose levels affect GSH in type 2 diabetes patients within the Malay race.
Methods: This study was observational with case-control, involving 25 patients with uncomplicated type 2 diabetes (receiving metformin and/or glimipiride) and 25 healthy controls. Random blood glucose levels were determined using ACCU-CHECK® Kit. Blood GSH levels were determined by Sigma GSH Assay Kit.
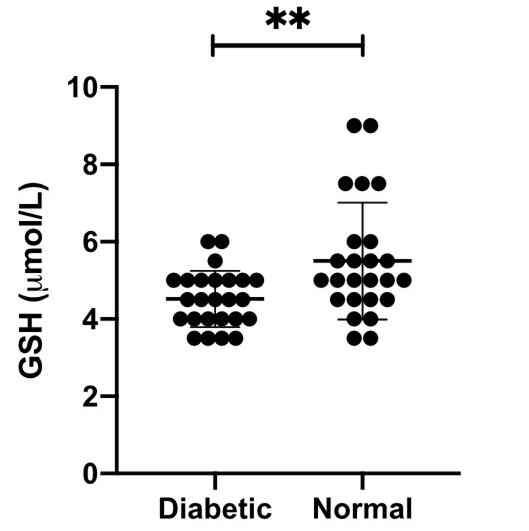
Results: Results show that type 2 diabetes patients have a significantly lower random blood glucose level compared with those of age-matched normal subjects (p<0.0001). Type 2 diabetic patients had significantly lower levels of GSH (p=0.00) than those of age-matched normal subjects. We found a moderate negative correlation (r=-0.437 and p=0.02) between the level of random blood glucose and the level of GSH.
Conclusion: The depletion of GSH during hyperglycemia may neutralize the free radicals indirectly generated by the abundant of glucose.
References
Ligita T, Wicking K, Francis K, Harvey N, Nurjannah I. How people living with diabetes in Indonesia learn about their disease: A grounded theory study. PLoS One. 2019;14: e0212019. https://doi.org/10.1371/journal.pone.0212019
Sugiarta IGRM, Darmita IGK. Profil penderita Diabetes Mellitus Tipe-2 (DM-2) dengan komplikasi yang menjalani rawat inap di Rumah Sakit Umum Daerah (RSUD) Klungkung, Bali tahun 2018. Intisari Sains Medis. 2020;11: 7. https://doi.org/10.15562/ism.v11i1.515
Kaura Parbhakar K, Rosella LC, Singhal S, Quiñonez CR. Acute and chronic diabetes complications associated with self-reported oral health: a retrospective cohort study. BMC Oral Health. 2020;20: 66. https://doi.org/10.1186/s12903-020-1054-4
Dludla PV, Joubert E, Muller CJF, Louw J, Johnson R. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-β-D-glucoside. Nutr Metab (Lond). 2017;14: 45. https://doi.org/10.1186/s12986-017-0200-8
Ohiagu FO, Chikezie PC, Chikezie CM. Pathophysiology of diabetes mellitus complications: Metabolic events and control. Biomed Res Ther. 2021;8: 4243–4257. https://doi.org/10.15419/bmrat.v8i3.663
Marcovecchio ML, University of Cambridge, Cambridge, UK. Complications of acute and chronic hyperglycemia. US Endocrinol. 2017;13: 17. https://doi.org/10.17925/USE.2017.13.01.17
Vodošek Hojs N, Bevc S, Ekart R, Hojs R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants (Basel). 2020;9. https://doi.org/10.3390/antiox9100925
Turpin C, Catan A, Guerin-Dubourg A, Debussche X, Bravo SB, Álvarez E, et al. Enhanced oxidative stress and damage in glycated erythrocytes. PLoS One. 2020;15: e0235335. https://doi.org/10.1371/journal.pone.0235335
Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J. 2016;24: 547–553. https://doi.org/10.1016/j.jsps.2015.03.013
Kristina H, Sartono N, Rusdi R. Kadar peroksida lipid dan aktivitas superoksida dismutase serum darah pada penderita diabetes melitus tipe 2. bioma. 2015;11: 1. https://doi.org/10.21009/Bioma11(1).1
Li J, Jiang R, Cong X, Zhao Y. UCP2 gene polymorphisms in obesity and diabetes, and the role of UCP2 in cancer. FEBS Lett. 2019;593: 2525–2534. https://doi.org/10.1002/1873-3468.13546
Hu C-Y, Lu D-L, Wu T, Cheng S-L, Wu T-T, Wang S, et al. Glutathione-S-transferases M1/T1 gene polymorphisms and male infertility risk in Chinese populations: A meta-analysis. Medicine. 2019;98: e14166. https://doi.org/10.1097/MD.0000000000014166
Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27: 1212–1221.e3. https://doi.org/10.1016/j.cmet.2018.04.010
Farinha JB, Ramis TR, Vieira AF, Macedo RCO, Rodrigues-Krause J, Boeno FP, et al. Glycemic, inflammatory and oxidative stress responses to different high-intensity training protocols in type 1 diabetes: A randomized clinical trial. J Diabetes Complicat. 2018;32: 1124–1132. https://doi.org/10.1016/j.jdiacomp.2018.09.008
Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70. https://doi.org/10.26402/jpp.2019.6.01
Lutchmansingh FK, Hsu JW, Bennett FI, Badaloo AV, McFarlane-Anderson N, Gordon-Strachan GM, et al. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS One. 2018;13: e0198626. https://doi.org/10.1371/journal.pone.0198626
Pulungan AB, Afifa IT, Annisa D. Type 2 diabetes mellitus in children and adolescent: an Indonesian perspective. Ann Pediatr Endocrinol Metab. 2018;23: 119–125. https://doi.org/10.6065/apem.2018.23.3.119
Rehman K, Akash MSH. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked? J Cell Biochem. 2017;118: 3577–3585. https://doi.org/10.1002/jcb.26097
Bandeira S de M, Guedes G da S, da Fonseca LJS, Pires AS, Gelain DP, Moreira JCF, et al. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and SOD activity. Oxid Med Cell Longev. 2012;2012: 819310. https://doi.org/10.1155/2012/819310
Krauss H, Koźlik J, Grzymisławski M, Sosnowski P, Mikrut K, Piatek J, et al. The influence of glimepiride on the oxidative state of rats with streptozotocin-induced hyperglycemia. Med Sci Monit. 2003;9: BR389-93.
Freckmann G, Jendrike N, Baumstark A, Pleus S, Liebing C, Haug C. User performance evaluation of four blood glucose monitoring systems applying ISO 15197:2013 accuracy criteria and calculation of insulin dosing errors. Diabetes Ther. 2018;9: 683–697. https://doi.org/10.1007/s13300-018-0392-6
Kermani SK, Khatony A, Jalali R, Rezaei M, Abdi A. Accuracy and precision of measured blood sugar values by three glucometers compared to the standard technique. J Clin Diagn Res. 2017;11: OC05-OC08. https://doi.org/10.7860/JCDR/2017/23926.9613
Klatman EL, Jenkins AJ, Ahmedani MY, Ogle GD. Blood glucose meters and test strips: global market and challenges to access in low-resource settings. Lancet Diabetes Endocrinol. 2019;7: 150–160. https://doi.org/10.1016/S2213-8587(18)30074-3
Srimaekarat T. Capillary blood glucose screening (Accu-Chek Advantage) for gestational diabetes. J Med Assoc Thai. 2009;92: 1268–1272.
Paula JS, Braga LD, Moreira RO, Kupfer R. Correlation between parameters of self-monitoring of blood glucose and the perception of health-related quality of life in patients with type 1 diabetes mellitus. Arch Endocrinol Metab. 2017;61: 343–347. https://doi.org/10.1590/2359-3997000000222
Riyaz MSU, Rather MK, Koul PA. Diabetes in immigrant tibetan muslims in kashmir, north india. J Immigr Minor Health. 2018;20: 410–415. https://doi.org/10.1007/s10903-017-0558-8
Kumawat M, Sharma TK, Singh I, Singh N, Ghalaut VS, Vardey SK, et al. Antioxidant Enzymes and Lipid Peroxidation in Type 2 Diabetes Mellitus Patients with and without Nephropathy. N Am J Med Sci. 2013;5: 213–219. https://doi.org/10.4103/1947-2714.109193
Abou-Seif MA, Youssef A-A. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta. 2004;346: 161–170. https://doi.org/10.1016/j.cccn.2004.03.030
Copyright (c) 2021 Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.







